After years working with Quality as part of a big corporate organization and working with external partners across regulated and less regulated industries, and even now as an independent quality assurance consultant, I evidenced several approaches to quality management that are still coexisting now days.
One common barrier to have value added and efficient quality programs are the short-term view and the lack of integration with other functions.
It is common to hear quality assurance and quality control teams complaining about lack of resources to be able to keep up on constantly changing regulations and requirements on top of the frequent out of compliance, out of trends and out of specification investigations at their facilities and operations. In general, taking a closer look to the team’s structure in the full organization, you can identify redundance, rework, lack of integration, conflicting metrics, lack of trust, and fear. All these leading to poor governance and performance issues.
Digital transformation became a necessary journey that not all teams accompany with the same pace. Implementing Digital Quality Systems require multiple changes, capability increase, interphase with other systems, among others. Choosing the right platform is tricky, Digital quality system in addition to be a trusted repository of data should facilitate the work at operations. In many companies, Digital Quality Systems might be in place but if those are not fully integrated with operation needs and other compliance requirements (ie. Safety, legal) the imposed system becomes a bureaucratic burden. DQS should be well evaluated and it development and implementation should involve employees at several levels and functions.
All the above are few examples associated to the Maturity of the Quality System in place. Let’st alk more about it.
The Maturity Model adapted to Quality Management
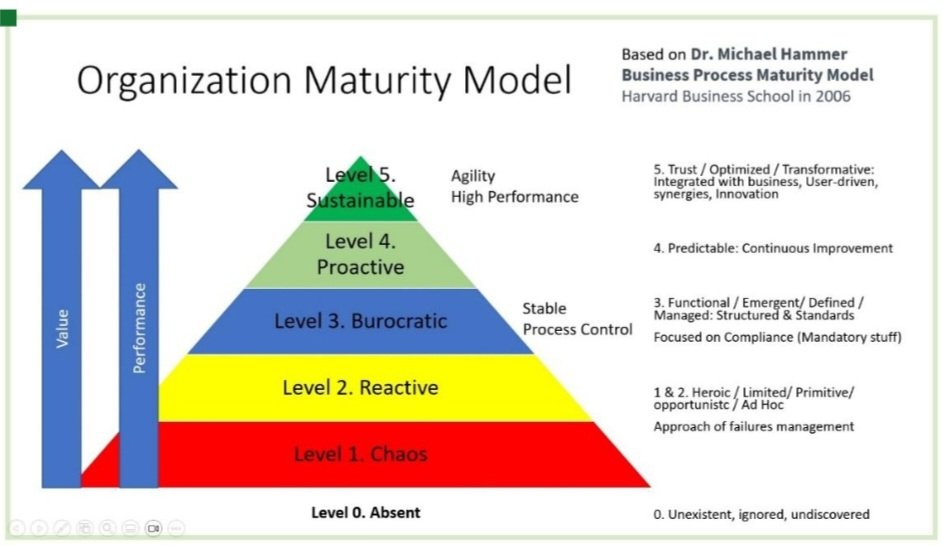
In the Model, level 1 and 2, found most frequently in less regulated businesses and new/small companies where issues and incidents are managed as come. In general, Companies that do not have a quality assurance team, focused on finished product quality control, strictly monitoring the minimum parameters as per local regulations. Poor risk assessment, resources testing and reporting, no trend analysis, no variation analysis, no rigor in process validation. Resources fighthing crisis most of the time despite having in place automation, sensors and state of the art technologies implemented in their operations. Resources not communicating efficiently among them, focused on teams metrics, and few or no resources are looking for transversal opportunities for sinergies and simplification. Everyone is very busy, learning is not a priority.
Level 4, already understood the need to work beyond compliance. Exceeding expectations. They need to be predictible, they need to monitor not only the minimum set of parameters required per regulation, they also need to understand the source of process variations, the repeated incidents, the root-cause of their issues, they conduct trend analysis of their data, learn from sister plants, and also watch-out for changing regulations in other markets and establish strategies to be ready before the change hit them. They are always ready for regulatory board inspections (no need to create SWAT teams for that purpose). GMP Certification is not a burden because is integrated to the ongoing business cycle. They understand what is the right thing to do and move ahead before is requested. They do not blame for the mistakes made, they learn from errors and action to minimize them. They involve and integrate other functions to simplify processes and be able to invest in more predictible approaches to assure stable, cost-effective and faster processes. Cost elimination is one of the positive outcomes of this strategy without compromising quality and employees satisfaction.
Level 5 is the high performing organization, the target of what organizations wants to achieve. Organizations are in continous improvement process, teams are working at the peak of their potential and are not feeling stagnated, individuals complementing to each other, not competing. Organization structure promote growth-mindset and inclusion. Results are beyond of the finantial gains, business metrics include sustainable development goals achieved according to a long-term vision. Increase Productivity is not about cutting people only, it is about increasing performance, it is having buffer for a timely response to unexpected changes, it is excellence in operations, people´s mastery and coordinated supply chain. All thanks to the integrated approaches across functions, and changed metrics from only financials to impact metrics. Everyone in the organization feel that is part and is contributing to the Company goals. Innovation is one of the main gains at this level.
The Enemies of the Maturity path: Case Examples Based on Real Experiences
Financial Focus – selling boxes.
An old health care distribution center, co-located in a manufacturing facility producing non-regulated products. DC distributing medicines made at sister manufacturing units from other countries, receiving and distributing OTC medicines from several sources, holding them until a contract laboratory, authorized by local government, finished the release testing according to local regulatory requirements. DC Quality Assurance resource managing also external laboratory, and local regulatory compliance. The DC quality manager totally disconnected from the local manufacturing operation (plant manager, site quality manager) which report to a different business unit. Connections with the global organization is poor, only when issues occurred. Working assumption: global approaches and standards will be accepted by local regulatory board, belief supported per history of past success. DC meeting expectations, selling the number of boxes committed, and agile to support additional innovation activities coming on the spot. Market is strategic but “small”. So, no investment is made to automatize and improve site quality management system. Everything is done manually. Years operating that way. Status Quo perceived as OK. Resources at the DC losing motivation, no career growth expected. Finally, hurdle with latest initiative because regulatory board did not accept the microbiological method suitability testing executed at the manufacturing operation following Company global standards. Local Regulatory Board expect suitability testing to be made locally following their own country Pharmacopeia requirements. After several rejected iterations with the regulatory board, the team proceed meeting the described local requirement. Initiative delayed one year.
Short sight / Short-term point of view
Manufacturing operating with “validated” water purification system, not reassessed after several years. Water monitoring performed at point of use; results look Okay. Risk assessment process identified some opportunities in some of the systems (exit of the Reverse Osmosis with high microbial counts, however, last point of purification CDI, just before storage and distribution, within limit). No action is done because changing the RO require stop of the operations. Suddenly, the OTC product manufactured with that water start to appear with increasing bioburden delaying product release, until finally three weeks later reaches Out of Specification limit. Production stopped, global capability engaged to assess, rescue the operations, and enable restart of operations as soon as possible. With the external eyes, multiple high-risk conditions identified at the operations and at the quality control laboratory. Operation stopped for several months to be able to correct corrosion points, dead legs, lack of capability of the team members, among other issues. USD millions lost.
Productivity without strategy, minimum investment
Productivity metrics based on people´s cut prevailing causing resources burn-out at operations and laboratory.
Preventive and Autonomous Maintenance Program optimized to do the minimum as per cost-saving metrics not linked to Quality program. Capacity at microbiology laboratory equipment not sufficient to avoid cross-contamination, night shift unsupervised. Operations not trusting lab results. Retesting, multiple investigations. Slowing release to the market. Customers dissatisfaction. Competition gaining market share.
Lack of Capability
Original analyst fully trained, promoted to another function due to the good results. Next generation of analysts responsible of the training of the new resources. Optimized training through virtual setting, minimum real supervised practice at the laboratory. Initial qualification period simplified and reduced from months to one week. Flow to the Work approach – chemist doing microbiology work and vice versa regardless their academical background or previous experience. Trained on the task. Metrics aligned with what is expected: Training records at the organization showing 90% capability. Consequences: Newest analysts learn imitating the ones preceding them, not able to solve problems, they do not understand what they are doing. Troubles knocking at the door.
Action after the fact
Waiting for the market intervention, for the recall, and celebrating how good is to do timely recall and communication. Not regretting enough not being acting in time through a rigorous risk assessment process to avoid the market intervention and the concomitant consumer/patient risk. Priority given to critical risk remediation, lack of effective continuous improvement process.
People Capability system – Fixed-mindset vs Growth mindset
Career path systems should be flexible enough to enable people to experience several functions in the manufacturing operations and across functions/organizations without jeopardizing technical mastery. Those career paths based on the belief that technical people are not good managers and vice versa are not helpful. Having highly qualified people trapped in poorly recognized/poor salaries technical career paths as pharmacologist and engineers doing only hidden laboratory work. Fix-mindset systems leading to demotivation, poor involvement with Company goals, high level of attrition.
Conclusion
Quality Assurance is not only about Quality Control. Quality Assurance is not only about compliance. If compliance is your target be prepared to be in the crisis mode because you are not going to be prepare for the changing regulation dynamics.
A benchmark quality assurance program has strategic planning completely aligned with Company vision were people capability, compliance, safety, and product performance (fully tested at the design phase by the research and development team) are integrated and in constant continuous improvement virtuous cycle. Loss elimination is not only financial but include impact metrics associated to sustainability goals. Quality without sustainability is incomplete. Sustainability and impact metrics is what will give to the strategy the great vision that inspire others to move forward and make the decision to make changes to be better. Perfection does not exist but can be a good inspiration to be better each time. Never conform yourself and your organization with the status quo. Improvement doing the right thing is the goal.
References
Stachowiak, A and Oleskow-Szlapka J (2018). Agility Capability Maturity Framework. 28th International Conference on Flexible Automation and Intelligent Manufacturing (FAIM2018), June 11-14, 2018, Columbus, OH, USA. Procedia Manufacturing 17: 603-610).
Glogovac M, Ruso J & Maricic M (2022). ISO 9004 maturity model for quality in industry 4.0. Total Quality Management & Business Excellence, 33:5-6, 529-547

Beatriz graduated in Biology in the Universidad Central de Venezuela (UCV), PhSc in Physiology and Biophysics by Instituto Venezolano de Investigaciones Científicas (IVIC) – Caracas, Venezuela; and Postdoc in Biophysics at Univ. of Califórnia (UCLA), Los Angeles (USA).
23+ years of work experience in Industry at Procter & Gamble, having global and regional responsibilities in the areas of Industrial Microbiology (R&D and manufacturing), Open Innovation, and quality assurance. Before P&G. worked also in academy and research during 13 years. Teaching experience (3 years) delivering courses for graduate and post-graduate students at UCV and IVIC.
Currently, delivering services as independent consultant in Quality Assurance, Industrial Microbiology, Open Innovation and Coaching at the BMRV Consulting (https://bmrvconsultoria.com/); active member of the Innovation Commitee of ABIHPEC (Brasilian Association of Industries of higiene, personal care, perfumery and cosmetics), and member of the Global Chamber, among others.








